Empirical Formula of Ascorbic Acid
The multiple is found by comparing the formula weight of the empirical formula with the molecular weight. QA By tamdoan January 19 2022 0 Comment what is the empirical.
Vitamin C Molecule Model And Chemical Formula Ascorbic Acid Ascorbate Skeletal Formula And Molecular Structure Vitamin Found In Various Foods Stock Photo Alamy
Multiplier molar mass empirical weight 17612g empirical weight g empirical weight 3 x atomic wt.
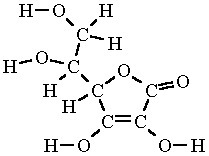
. Ascorbie acid vitamin C a white crystalline solid that is present in fruits and vegetables curves scury and may help. In one combustion analysis 524 g of ascorbic acid yields 786 g CO2 and 214 g H20. 4092 12 341 mol 340625 1001 1 H.
In 100 g of ascorbic acid we will have 4092 g C 458 g H and 5450 g O. For example in Sample Exercise 312 we saw. Given that the pseudoformula is c3407h453o3406.
Show transcribed image text. What is the empirical formula of ascorbic acid. What is the empirical of ascorbic acid.
C 4 x atomic wt H 3 x atomic weight O empirical weight. What is the empirical formula of ascorbic acid given that the pseudoformula is C3407H453O3406. The whole-number ratio gives us the subscripts for the empirical formula.
Empirical Formula of Ascorbic Acid By Bio_Destiney85 02 Sep 2022 Post a Comment Combustion Analysis Ex Find The Empirical Formula Of Vitamin C Ascorbic Acid A. Answer 1 of 3. Atoms First The empirical formula and the molecular formula of the ascorbic acid should be identified when the combustion of 28 1g ascorbic acid produces.
Determine the molecular formula of the dye. A CHO B CH2O C C2H3O2 D C3H4O3 E none of the above. Mol O the empirical formula is C3H4O32 Apr 2022 What is the empirical molar mass of ascorbic acid.
Answer 1 of 3. Ascorbic acid vitamin C contains C H and O. Determine the empirical formula of ascorbic acid.
Simplest or empirical formula. Determine the empirical formula of ascorbic acid if it is composed of 4092 carbon 458 hydrogen and 5450 oxygen. The dye has a percent composition of 7595 C 1772 N and 633 H by mass with a molar mass of about 240 gmol.
Be sure to answer all parts. 545 16 340625 mol 340625 1 Now as you. What is the empirical formula of ascorbic acid given that the pseudoformula is C 341 H 453 O 341.
In 100 g of ascorbic acid we will have 4092 g C 458 g H and 5450 g O. Since nCnHnO is 3 mol C4 mol H3. Express answer as a chemical formula.
The whole-number ratio gives us the subscripts for the empirical formula. 458 1 458 mol 340625 1345. Express your answer as a chemical formula.
General Chemistry I Solving For An Empirical Formula Youtube
Pin On Typography Design Handwritten
Ascorbic Acid Vitamin C Structural Chemical Formula And Molecule Model Vector Illustration Stock Vector Image Art Alamy
Ascorbic Acid C6h8o6 Structure Molecular Mass Properties Uses
Comments
Post a Comment